T-vant: A Novel Adjuvant for Transformative Vaccines
Inducing Immunity at the Source of Infection

Inducing Immunity at the Source of Infection
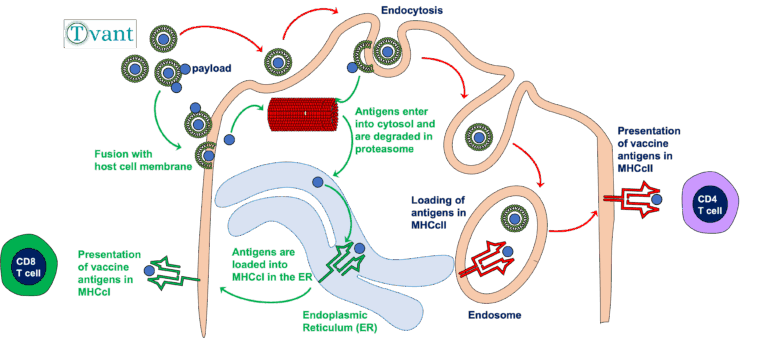
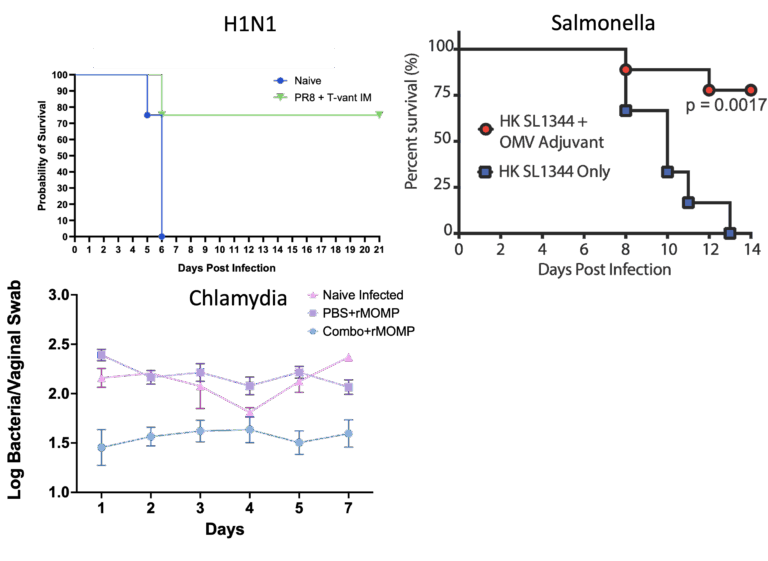
T-Vant is a potent adjuvant developed by Tulane scientists. This isolated lipid nanoparticle can stimulate not only the B-Cells and CD4+ T-Cells usually associated with vaccine protection, but also stimulates a potent CD8+ T-cell response. This new technology is suitable for use with any microbe or antigen and has been tested in multiple virus and bacterial challenge models.
Researchers

Why T-vant?
• Robust Multi-Arm Immune Boost
Activates humoral, cellular and mucosal responses, delivering broader, longer-lasting protection.
• Universal Vaccine Compatibility
Works as a simple admix with protein, peptide, inactivated, and other antigens, reducing need for reformulation.
• Dose-Sparing & Single-Shot Potential
Enhances neutralizing titers ≥10-fold and prolongs memory B- and T-cell responses, enabling lower antigen doses or single-dose regimens.
• Clean Safety & Low Reactogenicity
Demonstrates excellent tolerability in mice, rabbits, non-human primates, and the MIMIC human surrogate system.
• Mucosal & Needle-Free Delivery
Retains full adjuvant potency when given intranasally or orally, supporting needle-free mass-vaccination.
Publications
Other resources
| Title | Country | Date of Application | Serial / ID Number | Additional Notes |
|---|---|---|---|---|
| Bacterial Outer-Membrane Vesicles Derived Adjuvants | US | 10/09/2020 | 11,534,486 | |
| Bacterial Outer-Membrane Vesicles Derived Adjuvants | PCT | 04/10/2019 | PCT/US2019/026769 | Nationalized to "CA, IN, AU. KP. EPO. CN |
Schedule a call
Set up a meeting with one of our team members by using the Calendly integration below.
Is it urgent? Call us:
James Zanewicz (+1 504.919.3800),
Alexis Ducote (+1 337.540.4025),
Carolyn Scofield (+1 504.881.4542).