Tuleviate: A Safer, Non-Addictive Analgesic
Potent Pain Relief Without the Risk of Addiction or Tolerance

Potent Pain Relief Without the Risk of Addiction or Tolerance
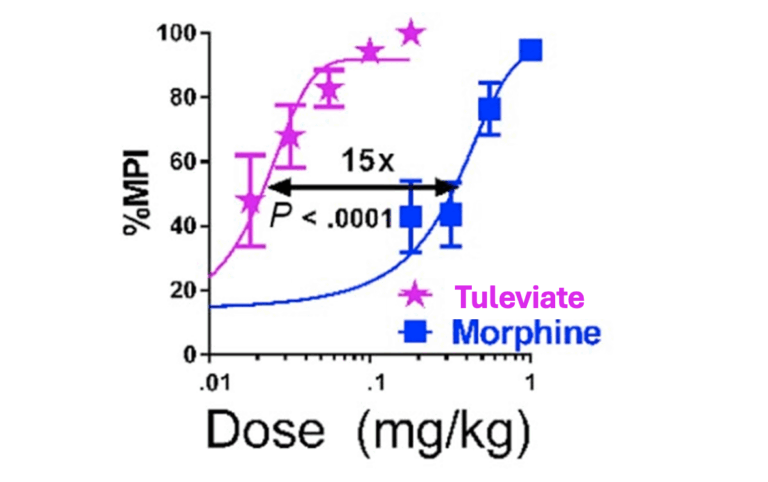
Tuleviate is a next-generation analgesic that selectively harnesses the body’s natural pain-relief pathways to deliver potent, long-lasting relief with a dramatically safer profile than traditional treatments. It is effective across a wide range of pain types, including acute, inflammatory, neuropathic, visceral, and postoperative pain.
Researchers
Why Tuleviate?
• Potent, Long-Lasting Pain Relief
Effective across acute, inflammatory, neuropathic, visceral, and postoperative pain models.
• Low Risk of Addiction
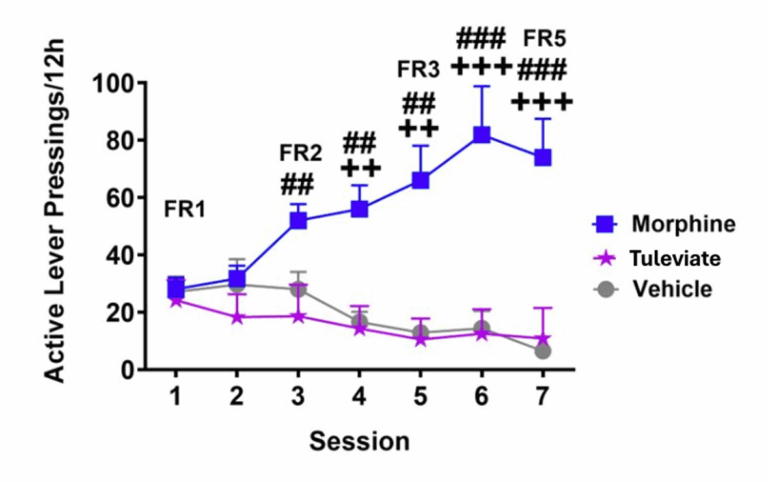
Demonstrates a non-addictive reward profile in preclinical models, unlike morphine or oxycodone.
• Minimized Withdrawal and Relapse
Blocks withdrawal symptoms and prevents relapse in models of opioid dependency.
• Reduced Tolerance Development
Maintains strong analgesic effects over time without the need for increasing doses.
• Avoids Activation of CGRP and Glial Cells
In contrast to chronic morphine, does not induce these two major causes of opioid tolerance and persistent pain.
• Safe and Well-Tolerated
Exhibits minimal respiratory depression, low toxicity, and a clean safety profile even at therapeutic doses.
Publications
Other resources
| Title | Country | Date of Application | Serial / ID Number | Additional Notes |
|---|---|---|---|---|
| Tuleviate PCT | US, Japan, Canada, Australia, and Hong Kong | 07/08/2011 | PCT/US2011/043306 | |
| Tuleviate | EPO, US | 05/01/2020 | PCT/US20/31140 | Method of use for treating opioid use disorder |
| Tuleviate NextGen (Glycosylated Analogs) | Nationalized to US, AU, CA, JP, EPO | 05/30/2023 | PCT/US2023/23879 | GLYCOSYLATED CYCLIC ENDOMORPHIN ANALOGS |
| Tuleviate NextGen (Glycosylated Analogs) | US | 11/26/2024 | 18/869,591 | |
| Tuleviate NextGen (Glycosylated Analogs) | Australia | 12/19/2024 | 2023279699 | |
| Tuleviate NextGen (Glycosylated Analogs) | Canada | 11/27/2024 | 3,257,674 | |
| Tuleviate NextGen (Glycosylated Analogs) | Japan | 11/29/2024 | ||
| Tuleviate NextGen (Glycosylated Analogs) | EPO | 12/19/2024 | 23816646.6 |
Funding Sources


Schedule a call
Set up a meeting with one of our team members by using the Calendly integration below.
Is it urgent? Call us:
James Zanewicz (+1 504.919.3800),
Alexis Ducote (+1 337.540.4025),
Carolyn Scofield (+1 504.881.4542).