TuCeptin: Broad-Spectrum, Resistance-Proof Antimicrobial Peptides
Stopping Infections Where Traditional Antibiotics Fail

Stopping Infections Where Traditional Antibiotics Fail
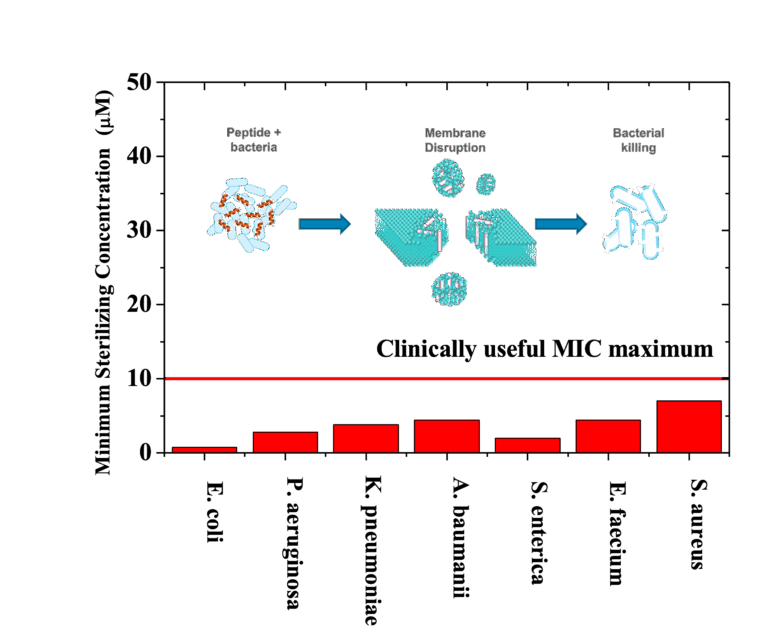
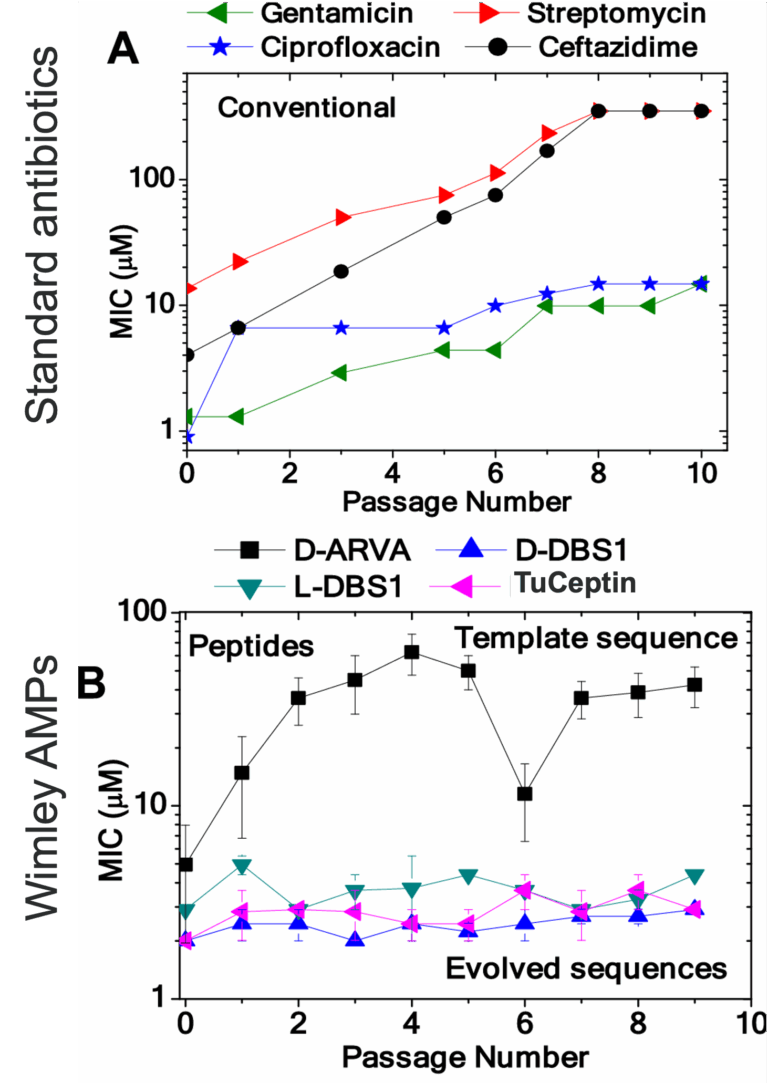
TuCeptin is a synthetically evolved antimicrobial peptide that sterilises all drug-resistant ESKAPE pathogens and their biofilms by rapidly lysing bacterial membranes . Unlike conventional antibiotics, it does not induce resistance or cross-resistance even after serial passage . D-amino formulations remain stable for more than five years lyophilised and over a year in saline at 4 °C, giving TuCeptin the shelf-life required for real-world deployment . In vivo, the peptide eradicates complex deep-wound infections caused by MRSA and MDR P. aeruginosa and show minimal cytotoxicity in a five-day topical dosing study . Independent work further validated the platform, with oral TuCeptin killing MDR E. coli and cured mouse enteritis while remaining highly stable and non-toxic.
Researchers
Why TuCeptin?
• Resistance-Proof Killing Mechanism
Rapidly disrupts bacterial membranes, leaving no opportunity for genetic escape—no resistance detected after serial passage of MDR isolates.
• Broad-Spectrum & Biofilm Power
Sterilises all ESKAPE pathogens and dismantles stubborn biofilms that shield chronic wounds, catheters, and implants.
• Validated In Vivo Efficacy
Cures deep-wound MRSA and MDR P. aeruginosa infections, and an independent 2025 study showed complete clearance of MDR E. coli enteritis in mice.
• Multi-Year Shelf Stability
D-amino peptide formulations remain potent for >5 years lyophilised and >12 months in saline at 4 °C.
• Low Mammalian Toxicity
Exhibits minimal cytotoxicity and no adverse local effects during repeated topical or systemic dosing.
• Scalable Synthesis
Short solid-phase build and simple purification streamline manufacturing; robust IP portfolio protects composition and clinical uses.
Publications
Other resources
| Title | Country | Date of Application | Serial / ID Number | Additional Notes |
|---|---|---|---|---|
| Peptide Compositions and methods of use thereof | PCT | 02/12/2021 | PCT/US2021/018031 | Nationalized to EPO, USA, AU, BR, CA, CN |
Schedule a call
Set up a meeting with one of our team members by using the Calendly integration below.
Is it urgent? Call us:
James Zanewicz (+1 504.919.3800),
Alexis Ducote (+1 337.540.4025),
Carolyn Scofield (+1 504.881.4542).